101.
A blanket protects ice from melting.
How?
Ans:
Ice melts when heat from outside reaches it. Under open air,
heat easily reaches them and so they melt easily. However when a
blanket is used, heat is prevented to a great deal because
blankets are poor conductors of heat. If an ice block is covered
with blankets, it does not allow heat to pass, from outside,
into the ice block according to the relation
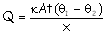 .
Thus, ice is protected from melting.
.
Thus, ice is protected from melting.
102. Why
are cloudy nights warmer than clear nights ?
Ans:
At night heat energy that the earth gained at the
day time is flowing out of (being lost from) the earth. On clear
nights, they are taken away by the air present by radiation and
convection mainly as well as some conduction (infinitesimally).
So they are much colder. However, clouds are bad conductors of
heat. When they are present, the conduction part is prevented.
Due to their presence, convection is also reduced a great deal
leaving only the radiation part. This is more like blocking the
heat radiated by the earth’s surface. Therefore, cloudy nights
become warmer than clear nights.
103. Two
layered window pane of equal thickness keeps a room warmer than
a single layered window pane of double thickness.
Ans:
The main idea behind using two layers of window panes is
trapping some air in between. The air trapped between those two
layers act as insulator of heat, so double layered pane window
becomes a bad conductor as compared to a single layer of double
thickness. Besides, due to lack of space, the air cannot undergo
convection, which erases any chance of leakage of heat from the
room to outside. Thus, the room will be warmer.
104. A
rough and blackened vessel is preferred for cooking. Why?
Ans:
During cooking, heat energy flows from the heat source to the
vessel and then to the materials inside. For that the vessel
should have a capability to absorb heat. This is possible if the
body is black because black substances have a higher capability
to absorb heat. Besides when they are made rough, multiple
reflections occur in those rough surfaces, absorbing some heat
in each of the reflections. After many reflections, heat energy
is almost absorbed all, which is then transferred to the food
materials to be cooked.
105. In
winter, metallic objects feel cooler than wooden ones. Why?
Ans:
Metals are good conductors of heat, whereas wood
is a poor one. When metallic objects are touched, they ‘conduct’
away heat from the body. When there is loss of heat, the body
feels cold at the region of touch. However, when wood is
touched, wood does not accept heat from the body, because it is
not a conductor. So the body heat remains preserved (no loss)
and so no feelings of coldness. When Due to the same reason,
metallic objects appear hotter than wooden ones when kept in the
sun.
106. Why
do we prefer woollen clothes to cotton clothes in winter?
Ans:
The
main idea is the trapping of air.
Although, both wool and cotton have
nearly the same thermal conductivity, the woollen clothes can
trap more air. Since air is a bad conductor, heat is preserved
in the body. In case of cotton clothes, no air is trapped, so
heat escapes out easily.
107. Birds
puff up their feathers on a cold day. Why?
Ans: On a cold day, there is more chance
of losing body heat to the outer environment due to large
temperature differences. The natural mechanism for reducing this
flow is inserting a medium of insulator medium in between. For
this they spread their feathers, which increase the space there,
causing air to rush into it. Since air is an insulator it
decreases the conduction of heat to the outside and preserves
their body heat from escaping out.
108. Why
are ventilators placed near the ceiling of a room?
Ans: The function of a ventilator is
throwing out of impure air (which is generally warmer than fresh
air due to its composition), so that fresh air can enter the
rooms. Warm air has a tendency to rise because of its low
density. When it finds the ventilator windows, it escapes
easily. The void space left by it is filled by fresh air (which
is generally cold) entering through the doors & windows placed
comparatively at lower height than ventilators. Thus, the room
will obtain continuous fresh air.
109. Why
is snow a better insulator than ice?
Ans: Ice is generally a single piece,
whereas snow is made of a lot of small pieces of ice particles
placed loosely. So it includes air particles in between. Since
air is one of the poorest conductors, the overall thermal
conductivity decreases. So snow is a better insulator than ice.
110. Wood
piece is a better conductor than saw dust of the same wood. Why?
Ans: Sawdust is made of wood particles
with air molecules in between. Wood is a poor conductor, but air
is poorer yet. Air being bad conductor of heat increases the
insulation. So, saw dust becomes an insulator naturally. So wood
will be a better conductor than its dust.
111. At
what condition will a piece of metal and a piece of wood feel
equally hot to the touch?
Ans:
Since metal is a good conductor of heat gains or
loses heat at higher rate than wood. Therefore, metals are found
to be hotter at higher temperature and colder at lower
temperature than a piece of wood at the same temperatures.
When the temperature of metal and wood is equal to the body
temperature, there will be no net heat transfer between human
body and metal piece or wood. Therefore, they would feel equally
hot to the touch.
112. The
thermal conductivity of felt is greater than air but felt is
used for thermal insulation. Why?
Ans:
Air is a bad conductor, but being a fluid, it
undergoes convection, which results in at least some loss of
energy. Felt is also a bad conductor like air. Moreover, it is a
solid. So there is no chance of convection and no
heat is lost by convection. Thus, felt provides better
insulation than air.
113. Why
does a piece of paper wrapped tightly on a wooden rod burn
quickly when held over aflame than a similar piece wrapped on a
metal rod?
Ans:
Before wood or any other substance burns, they should reach a
certain temperature often called as the Ignition Temperature,
for which there should be a proper storage of heat energy. Wood
is a bad conductor of heat, so it does not conduct away the heat
absorbed by paper, so paper quickly reaches up to the ignition
temperature, so it starts to burn. But metal is a good
conductor, so it conducts away the heat absorbed by paper. The
paper will constantly be losing its heat energy. So, paper takes
a longer time to reach the ignition temperature.
114. How
can water be boiled in a
paper cup, without burning the paper?
Or
How is boiling of water in a paper cup possible without
burning the cup?
Ans:
The boiling of water requires 100 0C
whereas the burning of paper requires its ignition temperature
which is greater than 100 0C. When water is heated in
paper cup, heat goes to the paper as well as water, so the
temperature of water as well as the cup rise to 100 0C
first. Then water starts to boil. At that time the ignition
temperature of paper is still not arrived yet, so it does not
burn. As the process of boiling goes on, a condition is reached
when water finishes off. Then only all the heat is directed to
paper, raising its temperature heavily and ultimately burning
it.
115. Although
aluminium is a good conductor of heat, how can an aluminium foil
with shiny surface be used to keep food hot for longer time?
Ans:
The phenomenon of conduction starts once the heat enters the
body, not before entering. When aluminium foils are used, they
reflect most of the radiation falling into them, because of
their shine. The reflected heat, when the foil is used around
food materials, goes back to the food itself. So loss of heat
from the food is prevented and the food would remain warm for
long. The shiny surface reflects heat radiations falling on it,
thus prevents the heat from food being lost and keeps the food
hot for a long time.
116. Hot
water pipes used in rooms are painted black. Why?
Ans:
Black surface is a good absorber, and also a good
radiator. In the hot water piping systems, they should be able
to radiate heat easily and sufficiently once they get heated. So
they are painted black to radiate heat to the room at a faster
rate which keeps the room warm.
117. Why
are white clothes more comfortable in summer than black ones?
Ans:
In summer the weather is hot which makes any gain
of heat from outside very uncomfortable. So clothes are to be
worn such that heat do not enter the body. This is achieved by
wearing white clothes. White objects reflect all radiation
falling on it. But, black objects absorb all radiation falling
on it. Therefore, white clothes are suitable in summer than
black ones. Due to the same reason, white umbrella is better
than black ones on hot days.