81. Two
different gases have exactly the same temperature. Does this mean that their
molecules have the same r.m.s. speed?
Solution: When the two gases
have exactly the same temperature, the average kinetic energy per molecule (= ˝
mc2 = 3/2 kT) for each gas is the same. But as the different gases
have molecules of different masses, the r.m.s. speed (c) of different gases
shall be different.
82.
83. Though velocity of air
molecules is about 0.5 kms-1 (about 484 m/sec.), the smell of scent spreads at a much
slower rate. Why?
Solution: This is because
molecules do not travel uninterrupted. They have random motion. The scent vapor
molecules undergo a number of collisions and trace a zigzag path. This is why
their effective displacement per unit time is low and spread is at a much
slower rate.
84. A gas is filled in a
cylinder fitted with a piston at a definite temperature and pressure. Explain on
the basis of kinetic theory, the pressure of the gas increases by raising its
temperature.
Solution: On raising the
temperature, the average kinetic energy and so average velocity of the gas molecules increases. As a result of
which more molecules collide with the wall of the cylinder per second and hence
greater momentum is transferred to the wall per second. Due to these
reasons, the average force will be more and so the pressure increases.
85. A gas is filled in a
cylinder fitted with a piston at a definite temperature and pressure. Explain on
the basis of kinetic theory: on pulling the piston out, the pressure of gas
decreases.
Solution: On pulling the
piston out, the volume of the cylinder for the given gas increases. Due to which
the molecules of gas get more space to move about. As a result of which, less
molecules will collide with the wall of cylinder per second and hence less
momentum is transferred to the wall per second. In addition to it, now these
collisions take place on the larger area of the walls. Due to both these
reasons, the pressure decreases.
86. On reducing the volume of
the gas at constant temperature, the pressure of the gas increases. Explain on
kinetic theory.
Solution: On reducing the
volume, the space for the given number of molecules of the gas decreases i.e.
no. of molecules per unit volume increases. As a result of which more molecules
collide with the walls of the vessel per second and hence a larger momentum is
transferred to the walls per second. Due to which the pressure of the gas
increases.
87. Under what conditions do
the real gases obey more strictly the gas equation, PV = RT? Explain.
Solution: The basic
properties of the molecules of an ideal gas are (i) zero size of the molecules
and hence zero volume of the molecules and (ii) no mutual intermolecular forces
between them. At low pressure, the volume of the given gas becomes large.
Therefore the volume of the molecules becomes negligible in comparison to the
volume of the gas. At high temperature, the molecules have large K.E. and so the
effect of the intermolecular force on the motion of the molecules becomes
negligible. Hence at low pressure and high temperature the real gas behaves as
ideal gas and gas equation is obeyed.
88. The absolute temperature
of a gas is made four times. How many times will its total kinetic energy
become? Root mean-square velocity of its molecules? Pressure?
Solution: K.E. per molecule
of a gas is directly proportional to temperature, hence K.E. will become four
times. R.M.S. velocity is directly proportional to the square root of
temperature, hence r.m.s. velocity becomes twice. Pressure is directly
proportional to (r.m.s. velocity)2. Hence pressure becomes 4 times.
89. The climate of a harbour
town is more temperature (i.e. without extremes of heat and cold) than that of a
town in a desert at the same latitude. Why?
Solution: This is because in
a harbour town, the relative humidity is more than in a desert town. Hence the
climate of a harbour town is without extremes of hot and cold.
90. Why do electrons in
insulators not contribute to its conductivity?
Solution: A material conducts
heat because of free electrons is present inside it. Since in insulators, no
free electrons are present, it cannot conduct heat.
91. A brass tumbler feels much
colder than wooden tray on a chilly day. Explain why?
or
In winter,
metallic objects feel cooler than wooden ones. Why?
Ans: Metals are good
conductors of heat, whereas wood is a poor one. When metallic objects are
touched, they ‘conduct’ away heat from the body. When there is loss of heat, the
body feels cold at the region of touch. However, when wood is touched, wood does
not accept heat from the body, because it is not a conductor. So the body heat
remains preserved (no loss) and so no feelings of coldness. Then due to the same
reason, metallic objects appear hotter than wooden ones when kept in the sun.
92. If air is a bad conductor
of heat, why do we not feel warm without clothes?
Solution: Air might be a bad
conductor, but it is a fluid (gaseous state) and so is a good medium for
convection. When people are without clothes, the air around the body gets some
heat from the body, so gets energy and rise. The empty space is promptly filled
by other fresh air, which also receives heat energy and rise up. As this process
goes on, the body goes on losing energy, giving a feeling of coldness. But when
clothes are there, they enclose air within their layers, stopping the free flow.
When they do get heated up due to the transfer of body heat, but they cannot go
anywhere, so other cooler air also can not come there to take their space. After
some time, these air molecules also attain almost the temperature of the body
and so loss of heat stops.
93. Why is it hotter at the
same distance over the top of fire than in front of it?
Solution: There are two
mechanisms by which heat from fire reaches the neighboring locations, one is by
radiation and the main contribution is due to convection. When there is
radiation, heat is uniformly distributed around it. Whereas during convection,
heat is mainly directed upwards because when fire heats the air molecules the
first thing they do is rise up. Therefore the region above the fire gets heated
up. However at the sides, heat is received due to the process of radiation only
or by cooling and settling down of the already risen air. Hence, it is hotter
at the same distance over the top of a fire than in front of it.
94. On a hot day, a cat is
left in sunlight with all the windows closed. After sometimes, it is found that
the inside
of the car is considerably warmer than the air outside. Explain why?
Solution: Glass transmits
about 50% of heat radiation coming from a hot source like the sun but does not
allow the radiation from moderately hot bodies to pass through it. Due to this,
when a car is left in the sun, heat radiation from the sun get into the car but
as the temperature inside the car is moderate, they do not pass back through its
windows. Hence, inside of the car becomes considerably warmer.
95. The earth constantly
receives heat radiation from the sun and gets warmed up. Why does the earth not
get as hot as the sun is?
Solution: Due to the large
distance of the earth from the sun, it receives only a small part of heat
radiation radiates by the sun. Further, there occurs loss of heat from the
surface of the earth due to radiation and convection. Hence the earth cannot
become as hot as the sun.
96. Heating system based on
circulation of steam is more efficient in warming a building than based on
circulation of hot water. Explain why?
Solution: Steam at 1000C
possesses more heat than the same mass of water at 1000C because one gram of
steam at 1000C possesses 540 calories of heat more than that
possessed by 1gm.of water at 1000C. So when steam is used they have
and can deliver more heat than water at the same temperature. That is why heating systems based
on circulation of steam are more efficient than those based on circulation of
hot water.
97. Is it necessary that black
colored objects should be considered black bodies?
Solution: No, it is not
necessary that all black colored objects should be considered as black bodies.
The main requirement for being a black body is that it should absorb radiations
(when its temperature is less than others) without reflection or transmission,
and also radiate (when temperature is greater than others). For example, if a
bright tin box with a hole is considered, radiations entering the hole get lost
inside due to multiple absorption. If a black surface is taken which is highly polished, it will not
behave as a perfect black body. On the other hand, the sun, which is a shining
hot sphere, behaves as a perfect black body.
98. An ink dot on a cup of
porcelain appears dark. When the same cup is heated to a high temperature, the
dot becomes brighter than the rest of the cup? OR Will design on a hot china
plate kept in dark room appear to be darker or brighter than white surface.
Substantiate your answer with proper reasoning?
Solution: The ink dot appears
dark, because it is better absorber of heat radiation than the white porcelain.
When the cup is heated, the ink dot appears brighter. This is because good
absorbers are good radiators. The energy stored earlier is now released as
energy. So, more the storage, more the emission.
99. A body with large
reflectivity is a poor emitter. Explain why?
Solution: This follows from
the relation
r + a + t = 1
When reflectivity is large,
the value of "r" will be high. This will be at the cost of "a" (absorptance) and
"t" (transmittance). So such substances do not absorb more, so they will have
less energy to emit. On the other hand, a black body has less reflectivity, so
absorbs more, and therefore is a good emitter as well.
100. Animals curl into a ball,
when they feel very cold.
Solution: Amount of heat radiated by a
body is proportional to the surface area according to the relation for
a spherical body. Similarly heat conducted away from the body also depends on
the area of exposure as evident from the relation
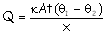 .
In addition when exposed area is less, less air molecules come in contact with
the body, which makes convection also less. All of these can occur if the
animals curl in to a spherical shape. So during winter, when the difference in
temperature of the body and outside is huge and the body tends to lose more
heat, they have to curl the body.
.
In addition when exposed area is less, less air molecules come in contact with
the body, which makes convection also less. All of these can occur if the
animals curl in to a spherical shape. So during winter, when the difference in
temperature of the body and outside is huge and the body tends to lose more
heat, they have to curl the body.