141. Explain why is steel or iron used widely in constructing
bridges?
142. Why are wires used in constructing bridges made of a
cluster of strings but not a single rod?
This phenomenon is related to the yielding stress. .
143. Why is there a split in the nib of a pen?
Ans: The
function of a pen is to ensure a uniform flow of ink to the
tip of pen for proper writing. In addition to uniformity, it
should be extremely slow for fine writing. The major
component of ink is water which wets other substance with
its high adhesive capacity and surface tension.
When a
split is made, it resembles a capillary tube. Since the
split has a small separation, it resembles small ‘r’. So
liquid slides along the tube away from the mass to other
locations according to the equation .
This will carry the ink to enough extent, i.e. up to the tip
(large ‘h’ due to small ‘r’).
144. Explain why is the pressure given by a mercury
barometer less than the actual value?
Ans:
The readings in a mercury barometer is the height of mercury the
atmospheric pressure can hold up. Mercury is exerting a
downward force (equal to its weight) whereas atmospheric
pressure tries to counter it (hold it up). But at the
surface, the phenomena of surface tension acts extra force
on the mercury mass. Mercury is a substance which has high
cohesive force, i.e. it wants to cling to itself. So there
is a net downward force on the surface at the very top,
which slightly reduces the height recorded. The reduction in
height due to surface tension is given by the relation
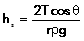 .
So the overall reading will be slightly reduced. (The
reduction will however be very small since the density of
mercury is very high).
.
So the overall reading will be slightly reduced. (The
reduction will however be very small since the density of
mercury is very high).
145. The ends of a glass tube become round on heating. Why?
Ans:
When heated, the glass tube melts slowly, changing into liquid
state. The surface then is acted by the surface tension
force (a characteristic of liquids) which makes the molecules be limited and as packed as
possible. This makes it take as minimum volume possible.
This is possible only if the surface becomes spherical, i.e.
round.
146. Can you use water instead of mercury in a barometer
to read atmospheric pressure?
Ans:
The process is impractical for two reasons. One is that
there is the need for a very long tube if water is used
(10.3 m for water instead of 0.76 m in case of mercury). The other is
the action of surface tension. In case of mercury the net
downward force due to surface tension is downwards which
reduces the reading slightly (because of high density). In
water, however, there is a net upward force. The increase in
this height due to surface tension is more because of less
density of water. So the reading will be very high than the
actual readings.
147. Why does a small pin when placed gently over the
surface of water floats?
Ans:
A pin is usually made of metals which definitely has a
higher density than water. So theoretically, it should sink.
However, when it is slowly placed on water surface, the
surface first depresses slightly, causing it to extend and
become tight slightly. So it comes under tension and, which
develops s tendency to return to initial state. This causes
a resultant upward force. Since water has high value of
surface tension than others, the resultant force is also
high and so it can overcome the effect of gravity which
holds the pin up. However, if it is placed abruptly, the
surface breaks and in no way that it can hold up the pin.
148. What happens to the surface tension of a liquid when
the temperature rises?
Surface
tension is due to mutual attraction of molecules, specially
the molecules of the surface in overall by the inner
molecules. when the temperature rises, the molecules show a
higher degree of motion because of increased kinetic energy.
This takes the molecules farther away from each other,
causing an increase in the distances in between them. So,
the attractive forces are slightly reduced. the effect of
this is the reduction of attraction inside as well as at the
surface. So the surface tension decreases.
149. Particles of camphor move around randomly on the surface of water.
Why?
Ans: Water is
a substance with very high surface tension, i.e. its surface
will act as a tough layer for any other substance placed on
it. When camphor is placed on it, camphor spreads on it
rapidly (with a very very high velocity compared to others).
The phenomena of spreading is random and not uniform. When
they spread, they give opposite force on the camphor piece.
In addition, the faces of camphor is very randomly oriented, So
when they spread and wherever they spread, the camphor will move in opposite
direction, causing very very random motion.
150.
How does a 'towel' work?
Towel is
made of cotton or any other fabric, which consists of very
thin fibers interwoven on each other. This causes the
formation of a lot of thin channels in between them which
act as the capillary tubes. So when a towel is placed over
wet surface, water climbs into these channels according to
the relation
151. When a chalk piece is immersed in water, why are bubbles
emitted?
Ans:
Chalk consists of a lot of fine pores in it which are
interconnected with each other to form thin channels. So
when it is immersed in water, water travels through the
channels according to the relation
152. Explain why are small water drops spherical in shape?
or
Explain why large drops are flat in shape?
Ans:
When water drops are smaller, the surface tension forces
along the surface, produce overall resultant forces towards
the center. This presses and packs the molecules as inward
as possible. This results in their assumption of a spherical
shape. At that time, since the mass involved is more, the
force of gravity (i.e. the downward force) is less.
However,
when the drop is large, the surface tension has to hold more
amount of molecules, which is itself tougher than in case of
small drops. Besides, the mass is now large, so the force of
gravity will be high. The molecules, instead of being
directed towards the center also receive a downward force.
so some molecules slide downwards, giving the whole drop a
flatter shape.
154. Water rises in the capillary but mercury gets depressed.
Explain why?
155. Why is a liquid in contact with a solid has normally a
curved surface?
156. Why is it easier to wash clothes in hot water or
soap solution or by the use of a detergent?
Ans:
The phenomena of washing clothes is related to the access of
water to dirt and other unwanted substances and carry them
away. This requires that water does not block itself and
others because of its high surface tension. This requires
that the surface tension of water be reduced, which can be
achieved by adding some foreign substances as detergents and
soap. Besides, the increase of temperature also decreases
the surface tension, so water can reach every region and
clean them.
157. Water spreads on a clean glass plate, while the mercury
on the same glass attains drops. Explain why?
Ans:
This phenomena has much to do with the dominance of the
adhesive or the cohesive forces. When water is placed on the
glass surface, the adhesive forces dominate over the
cohesive and so glass will try to attract as much water as
possible. So water spreads easily. on the other hand,
mercury has a high value of cohesive force compared to the
adhesive force, and so tries to avoid glass as much as
possible. Due to which it takes up a round shape in the form
of drops.
158. Why does addition of foreign substances in water
decreases its surface tension?
Water is one of the substance having greatest value of
surface tension, for which the attraction between their
molecules play the main role. When any foreign substance is
added, they come in between water molecules, which decreases
the overall attractive force between them. This reduction is
seen at the surface also and so the surface tension also
decreases. Addition of detergents and soaps in water takes
advantage of this phenomenon.
159. Capillary of insufficient height is dipped in a liquid.
Will the liquid over flow? Explain.
Ans:
No, and never.
The phenomenon is cleared by the following treatment.
These relations show that the radius of curvature of the
meniscus depends on the height available for it to expand.
Less the height available, more is the radius of curvature
of the meniscus. The maximum value 'R' can achieve is
infinite, when the height is 0, i.e. the glass tube is taken
to the level of the liquid. Below that there is no question
of overflowing.
160. Oil like kerosene, petrol is poured on stagnant water to
avoid mosquitoes attack. Does this mean that such oils are
poisonous to them?
Ans:
No, the phenomenon is more physical rather than chemical. Water
has high surface tension whereas petroleum has less. So when
they are poured on water they spread easily and completely
on water. This will make them form a uniform but thin layer
over water. When insects come to land on them or the eggs
fall on the surface, they get drowned because such layers
can not hold them (the reason is less surface tension). Once
they are drowned, the oxygen supply is cut off and this
kills them.